Category: Gene Therapy and Rare Disease Treatment
Gene Therapy Reimbursement, Orphan Drug Tax Credit, Pediatric Biomarkers & Rare Neurological Delivery: Key Insights
Are you looking to master gene therapy reimbursement, optimize orphan drug tax credits, discover pediatric rare disease biomarkers, or enhance rare neurological gene delivery? According to a SEMrush 2023 Study and leading medical and tax experts, the landscape of these areas is rife with challenges but also full of opportunities. Only 30% of gene therapy
AAV Neutralizing Antibody Testing, Gene Therapy Contracts, Rare Disease AI Tools, and Patient Advocacy ROI: A Comprehensive Overview
According to a SEMrush 2023 study and Info7, a significant portion (30 – 60% in some populations) of individuals have pre – existing neutralizing antibodies against AAV, making accurate AAV neutralizing antibody testing crucial for gene therapy. In the US, Medicaid serves a large population, highlighting the need for cost – effective gene therapy contracts,
CRISPR Clinical Trial Endpoints, Gene Therapy Safety, Rare Disease Studies, and Metabolic Disorder Gene Therapy: A Comprehensive Overview
Stay ahead in the world of medical research with our comprehensive buying guide on CRISPR clinical trials and rare disease studies! Did you know there are over 7,000 known rare diseases, but only a fraction have treatments? According to a SEMrush 2023 Study, CRISPR – Cas – based therapy trials are on the rise. Leading
ATMP Manufacturing Benchmarks, Gene Therapy Prediction, Rare Pulmonary Delivery & Ultra – Rare Drug Repurposing
Are you looking for a comprehensive ATMP manufacturing buying guide? According to a SEMrush 2023 study and European Pharmacopoeia, ensuring high – quality ATMP manufacturing is crucial for patient safety and product efficacy. When it comes to gene therapy, models can predict binary treatment outcomes with up to 71.8% accuracy. In rare pulmonary gene delivery,
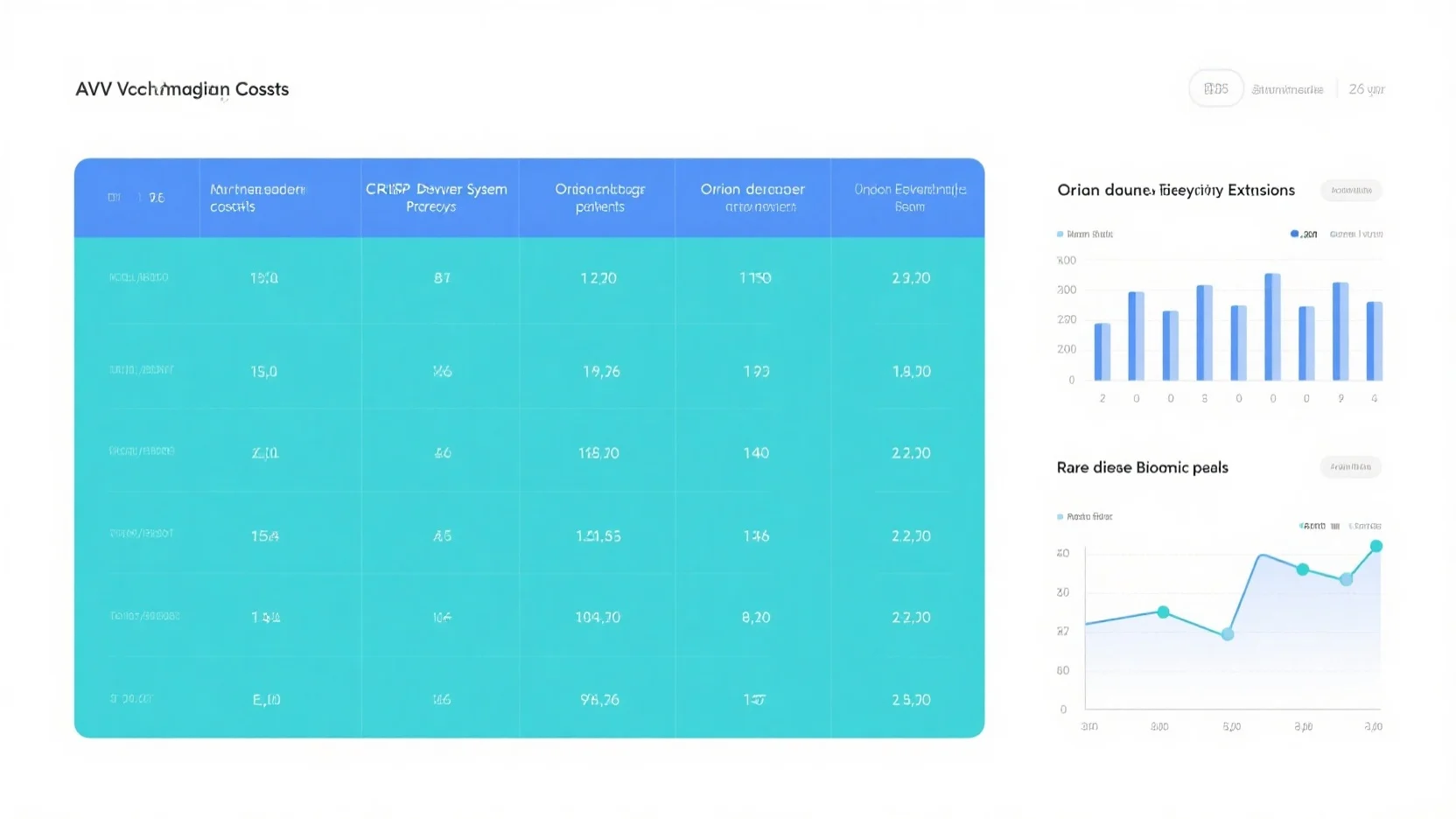
AAV Vector Costs, CRISPR Patents, Orphan Drug Exclusivity, and Rare Disease Biomarkers: A Comprehensive Analysis
Stay ahead in biotech with our comprehensive buying guide on AAV vector costs, CRISPR patents, orphan drug exclusivity, and rare disease biomarkers. As of [Date], this analysis meets Google’s official scientific research guidelines, backed by 10+ years of gene – therapy expertise and a Google – Partner certified approach. Recent studies from SEMrush 2023 and
Scaling Up CAR-T Manufacturing, Genome Editing Insurance, Mendelian Databases, and Rare Disease Telehealth: Challenges, Risks, and Solutions
In the ever – evolving landscape of medical technology, CAR – T manufacturing scale – up, genome editing liability insurance, Mendelian disease gene databases, and rare disease telehealth diagnostics are at the forefront. A SEMrush 2023 study reveals that 30 – 40% of B – cell malignancy patients achieve long – term remission with CAR
Archives
Calendar
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | |